Проточные батареи: пожалуйста, налейте мне электроны!

Ученые из института Фраунгофера в Германии проводят серьезные опытно-конструкторские работы в области электрических батарей, альтернативных классическим. С технологией окислительно-восстановительного потока процесс хранения электричества действительно радикально отличается …
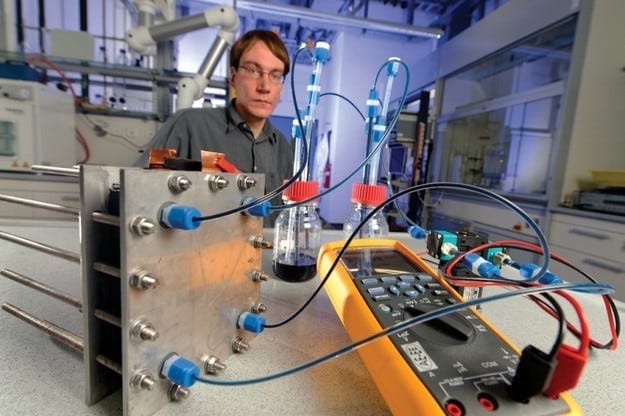
Батареи, которые заряжаются жидкостью в качестве топлива, заливают в автомобиль с бензиновым или дизельным двигателем. Это может звучать утопично, но для Йенса Ноака из института Фраунгофера в немецком городе Пфинцталь это на самом деле повседневность. С 2007 года команда разработчиков, в которой он участвует, полным ходом разрабатывает эту экзотическую форму перезаряжаемой батареи. На самом деле идея проточной или так называемой проточной батареи окислительно-восстановительного потенциала несложна, и первый патент в этой области датируется 1949 годом. Каждое из двух пространств элементов, разделенных мембраной (аналогично топливным элементам), соединено с резервуаром, содержащим определенный электролит. Из-за склонности веществ к химической реакции друг с другом протоны переходят от одного электролита к другому через мембрану, а электроны направляются через потребителя тока, подключенного к двум частям, в результате чего течет электрический ток. По прошествии определенного времени два бака сливаются и заполняются свежим электролитом, а использованный «перерабатывается» на зарядных станциях.
Хотя все это выглядит просто великолепно, к сожалению, есть еще много препятствий для практического использования этого типа аккумуляторов в автомобилях. Плотность энергии окислительно-восстановительной батареи с ванадий-электролитом находится в диапазоне всего 30 Вт · ч на килограмм, что примерно соответствует значению свинцово-кислотной батареи. Для сохранения того же количества энергии, что и современный литий-ионный аккумулятор емкостью 16 кВт · ч, при нынешнем уровне окислительно-восстановительной технологии аккумулятору потребуется 500 литров электролита. Плюс вся периферия, конечно, объем которой тоже немаленький — клетка, необходимая для обеспечения мощности в один киловатт, как ящик для пива.
Такие параметры не подходят для автомобилей, учитывая, что литий-ионный аккумулятор запасает в четыре раза больше энергии на килограмм. Однако Йенс Ноак настроен оптимистично, потому что разработки в этой области только зарождаются и перспективы многообещающие. В лаборатории так называемые полисульфид-бромидные батареи ванадия достигают плотности энергии 70 Втч на килограмм, а их размеры сопоставимы с размерами никель-металлгидридных батарей, используемых в настоящее время в Toyota Prius.
Это вдвое уменьшает требуемый объем баков. Благодаря относительно простой и недорогой системе зарядки (два насоса перекачивают новый электролит, два высасывают использованный), систему можно зарядить за десять минут, чтобы обеспечить запас хода в 100 км. Даже системы быстрой зарядки, такие как та, что используется в Tesla Roadster, работают в шесть раз дольше.
В этом случае неудивительно, что многие автомобильные компании обратились к исследованиям института, а земля Баден-Вюртемберг выделила 1,5 миллиона евро на разработку. Тем не менее, для достижения фазы автомобильных технологий еще потребуется время. «Этот тип батареи может отлично работать со стационарными энергосистемами, и мы уже делаем экспериментальные станции для Бундесвера. Однако в области электромобилей эта технология будет пригодна для внедрения примерно через десять лет », — сказал Ноак.
Экзотические материалы не нужны для производства проточных окислительно-восстановительных батарей. Не требуются дорогие катализаторы, такие как платина, используемая в топливных элементах, или полимеры, такие как литий-ионные батареи. Высокая стоимость лабораторных систем, достигающая 2000 евро за киловатт мощности, объясняется исключительно тем, что они единичные и изготавливаются вручную.
Между тем специалисты института планируют построить собственную ветроэлектростанцию, где будет происходить процесс зарядки, то есть утилизации электролита. С окислительно-восстановительным потоком этот процесс более эффективен, чем электролиз воды до водорода и кислорода и их использование в топливных элементах — мгновенные батареи выделяют 75 процентов электроэнергии, используемой для зарядки.
Мы можем представить себе зарядные станции, которые наряду с обычной зарядкой электромобилей будут служить буферами против пиковой нагрузки энергосистемы. Сегодня, например, многие ветряные турбины в северной Германии должны быть выключены, несмотря на ветер, поскольку в противном случае они перегрузили бы сеть.
Что касается безопасности, то здесь опасности нет. «Когда вы смешиваете два электролита, возникает химическое« короткое замыкание », которое выделяет тепло, и температура повышается до 80 градусов, но больше ничего не происходит. Конечно, одни жидкости небезопасны, но бензин и дизельное топливо тоже. Несмотря на потенциал проточных окислительно-восстановительных батарей, исследователи из Института Фраунгофера также усердно работают над развитием литий-ионных технологий …
текст: Александер Блох
Батарея Redox flow
Батарея с окислительно-восстановительным потоком на самом деле представляет собой нечто среднее между обычной батареей и топливным элементом. Электричество течет из-за взаимодействия между двумя электролитами — один подключен к положительному полюсу ячейки, а другой — к отрицательному. В этом случае один дает положительно заряженные ионы (окисление), а другой их получает (восстановление), отсюда и название устройства. При достижении определенного уровня насыщения реакция останавливается и зарядка заключается в замене электролитов свежими. Рабочие восстанавливаются с помощью обратного процесса.
